基本情况
姓名:朱庭顺
性别:男
出生年月:1983-08
籍贯:广东广州
职位:教授,博士生导师
联系方式
邮箱:zhutshun@mail.sysu.edu.cn
通讯地址:永利集团3044noc登录入口(南校区丰盛堂)B520
邮编:510275
个人网站:http:/zh-hans/teacher/779
教育经历
2009年9月-2012年7月,中国科学院上海药物研究所,理学博士
2006年9月-2009年7月,中国科学院上海药物研究所,理学硕士
2002年9月-2006年6月,南京大学,理学学士
工作经历
2017年7月至今,永利集团3044noc登录入口,教授,博士生导师
2013年2月-2017年7月,新加坡南洋理工大学(Nanyang Technological University),博士后(Research Fellow)
2012年7月-2013年2月,中国科学院上海药物研究所,助理研究员
讲授课程
《有机化学I》,《有机化学II》,《有机化学》慕课(https://www.icourse163.org/learn/SYSU-1206623843),《现代化学研究方法与技术》
科研方向
电化学反应诱导的新型反应,有机小分子催化的苯环构建,不对称催化与合成,天然产物、生物活性分子及其他功能分子的高效合成。
(长期招收博士后。欢迎对有机合成感兴趣的博士发邮件联系。)
获奖情况
ACP LECTURESHIP AWARD 2019
“芙兰.思源”奖教金 2019
上海市研究生优秀成果 2014
中国科学院优秀博士学位论文 2013
论著一览
24. “Atroposelective Arene Formation by Carbene-Catalyzed Formal [4+2] Cycloaddition,” Xu, K.; Li, W.; Zhu, S.; Zhu, T.* Angew. Chem. Int. Ed. 2019, 58, 17625
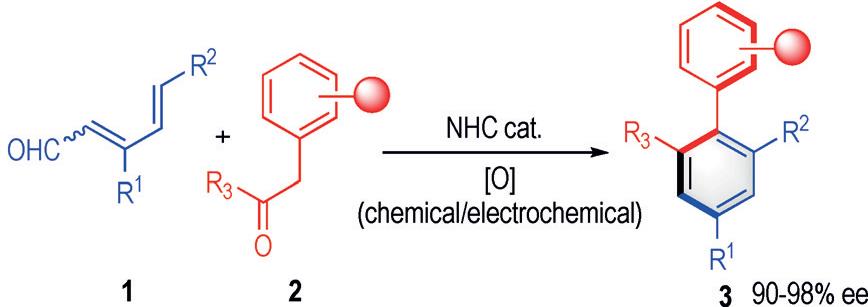
23. “Carbene-Catalyzed Desymmetrization and Direct Construction of Arenes with All-Carbon Quaternary Chiral Center” Zhu, T.§; Liu, Y.§; Smetankova, M.; Zhuo, S.; Mou, C.; Chai, H.*; Jin, Z.; Chi, Y. R.* Angew. Chem. Int. Ed. 2019, 58, 15778.
22. “Access to All-Carbon Spirocycles through a Carbene and Thiourea Cocatalytic Desymmetrization Cascade Reaction” Zhuo, S.; Zhu, T.; Zhou, L.; Mou, C.; Chai, H.; Lu, Y.; Pan, L.*; Jin, Z.; Chi. Y. R.* Angew. Chem. Int. Ed. 2019, 58, 1784.
21. “Stabilized Molybdenum Trioxide Nanowires as Novel Ultrahigh-Capacity Cathode for Rechargeable Zinc Ion Battery” He, X.; Zhang, H.; Zhao, X.; Zhang, P.; Chen, M.*; Zheng, Z.; Han, Z.; Zhu, T.; Tong, Y.; Lu, X.* Adv. Sci. 2019, 1900151
20. “Engineering channels of metal–organic frameworks to enhance catalytic selectivity” Liu, Y.; Shen, Y.; Zhang, W.*; Weng, J.; Zhao, M.; Zhu, T.; Chi, Y. R.; Yang, Y.; Zhang H.*; Huo, F.* Chem. Commun. 2019, 55, 11770.
19. “Carbene-catalyzed enantioselective oxidative coupling of enals and di(hetero)arylmethanes” Chen, Q.§; Zhu, T.§; Majhi, P. K.; Mou, C.; Chai, H.; Zhang, J.; Zhuo, S.; Chi, Y. R.* Chem. Sci. 2018, 9, 8711.
18. “Carbene and Acid Cooperative Catalytic Reactions of Aldehydes and o‑Hydroxybenzhydryl Amines for Highly Enantioselective Access to Dihydrocoumarins” Chen, X.; Song, R.; Liu, Y.; Oii, C. Y.; Jin, Z.; Zhu T.; Wang, H.; Hao, L.; Chi, Y. R.* Org. Lett. 2017, 19, 5892
17. “Carbene-Catalyzed Formal [5 + 5] Reaction for Coumarin Construction and Total Synthesis of Defucogilvocarcins” Huang, X.§; Zhu, T.§; Huang, Z.; Zhang, Y.; Jin, Z.; Zanoni, G.; Chi, Y. R.* Org. Lett. 2017, 19, 6188
16. “Green and Rapid Access to Benzocoumarins via Direct Benzene Construction through Base-Mediated Formal [4+2] Reaction and Air Oxidation,” Mou, C. §; Zhu, T. §; Zheng, P.; Yang, S.; Song, B.-A.*; Chi, Y. R.* Adv. Synth. Catal. 2016, 358, 707
15. “N-heterocyclic carbene-catalyzed δ-carbon LUMO activation of unsaturated aldehydes,” Zhu, T., Mou, C.; Li, B.; Smetankova, M.; Song, B-.A.*; Chi, Y. R.* J. Am. Chem. Soc. 2015, 137, 5658. (highlighted by Synfacts 2015, 11, 0767)
14. “Benzene construction via organocatalytif formal [3+3] cycloaddition reaction,” Zhu, T.; Zheng, P.; Mou, C.; Yang, S.; Song, B-.A.; Chi, Y. R.* Nature Commun. 2014, 5, 5027.
13. “Metal and carbene organocatalytic relay activation of alkynes for stereoselective reactions,” Namitharan, K.§; Zhu, T. §; Cheng, J.; Zheng, P.; Li, X.; Yang, S.; Song, B.-A.; Chi, Y. R.* Nature Commun. 2014, 5, 3982
12. “Indium-Mediated asymmetric intramolecular allenylation of N-tert- butanesulfinyl imines: efficient and practical access to chiral 3-allenyl-4-aminochromanes,” Su, L.; Zhu, T.-S.; Xu, M.-H. * Org. Lett. 2014, 16, 4118.
11. “N-heterocyclic carbene-catalyzed chemoselective cross-aza-benzoin reaction of enals with isatin-derived ketimines: access to chiral quaternary aminooxindoles,” Xu, J.; Mou, C.; Zhu, T.; Song, B.-A. *; Chi, Y. R. * Org. Lett. 2014, 16, 3272.
10. “Access to oxoquinoline heterocycles by N-heterocyclic carbene catalyzed ester activation for selective reaction with an enone” Fu, Z.; Jiang, K.; Zhu, T.; Torres, J.; Chi, Y. R. * Angew. Chem. Int. Ed. 2014, 53, 6506.
9. “β-Carbon activation of saturated carboxylic esters through N-heterocyclic carbene organocatalysts,” Fu, Z.; Xu, J.; Zhu, T.; Leong, W. W. Y.; Chi, Y. R. * Nature Chem. 2013, 5, 835.
8. “Rhodium-Catalyzed Enantioselective Addition to Unsymmetrical α-Diketones: Tandem One-Pot Synthesis of Optical Active 3-Quaternary Isochroman Derivatives,” Zhu, T.-S.; Chen, J.-P.; Xu, M.-H. * Chem. Eur. J. 2012, 19, 865. (Highlighted by Synfacts, 2013, 9, 0360).
7. “Chiral Sulfinamide-Olefin Ligands: swichable Selectivity in Rhodium-Catalyzed Asymmetric 1,2-Addition of Arylboronic Acids to Aliphatic α-Ketoesters,” Zhu, T.-S.; Xu, M.-H. * Chin. J. Chem. 2013, 31, 321.
6. “Rhodium-Catalyzed, Highly Enantioselective 1,2-Addition of Aryl Boronic Acids to α-Ketoesters and α-Diketones Using Simple, Chiral Sulful-Olefin Ligands” Zhu, T.-S.; Jin, S.-S.; Xu, M.-H. * Angew. Chem. Int. Ed. 2012, 51, 780. (Highlighted by Synfacts, 2012, 8, 0422).
5. “Efficient Synthesis of Optically Active α-Quaternary Amino Acids by Highly Diastereoselective [2,3]-Rearrangement of Allylic Ammonium Ylides,” Zhu, T.-S.; Xu, M.-H. * Chem. Commun. 2012, 48, 7274.
4. “Rhodium-Catalyzed Enantioselective 1,2-Addition of Arylboronic Acids to Heteroaryl α-Ketoesters for Synthesis of Heteroaromatic α-Hydroxy Esters,” Wang, H.; Zhu, T.-S.; Xu, M.-H. * Org. Biomol. Chem. 2012, 10, 9158. (Cover Article)
3. “Design of N-Cinnamyl Sulfinamides as New Sulful-containing Olefin Ligands for Asymmetric Catalysis: Achieving Structural Simplicity with a Categorical Linear Framework,” Jin, S.-S.; Wang, H.; Zhu, T.-S.; Xu, M.-H.* Org. Biomol. Chem. 2012, 10, 1764. (Cover Article) (Highlighted by Synfacts, 2012, 8, 0651).
2. “Design of Chiral Sulfoxide-Olefins as a New Class of Sulful-Based Olefin Ligands for Asymmetric Catalysis,” Qi, W.-Y.; Zhu, T.-S.; Xu, M.-H. * Org. Lett. 2011, 13, 3410.
1. “Synthesis and Characterization of Chiral Polymer Complexes Incorporating Polybinaphthyls, Bipyridine, and Eu(III),” Cheng, Y.*; Zou, X.; Zhu, T.; Liu, Y.; Zhang, S.; Huang, H. J. Polym. Sci. A: Polym. Chem. 2007, 45, 650.

